
What is Dissolution:
Dissolution is the process by which a solid substance enters into a liquid known as dissolution medium or solvent to form a solution.
Dissolution is a test which is used for a pharmaceutical product to evaluate the rate of release of a drug substance from the dosage form.
Dissolution test is performed for the Dosage form like Tablets, Capsules, Granules, Ointment and Creams etc. to check the percentage of drug release.
Drug dissolution in Body:
In the body, a pharmaceutical active ingredient must be in solution before it can be absorbed by the blood and ultimately carried to the receptor site to render a therapeutic effect.
Solid oral dosage forms typically begin to disintegrate and dissolve in the stomach and then the resulting solution passes into the small intestine where dissolution continues.
The dissolved active ingredient is absorbed into the blood stream through the walls of the small intestine.
Types of Dissolution Apparatus :
USP Dissolution Apparatus 1 – Basket
USP Dissolution Apparatus 2 – Paddle
USP Dissolution Apparatus 3 – Reciprocating Cylinder
USP Dissolution Apparatus 4 – Flow-Through Cell

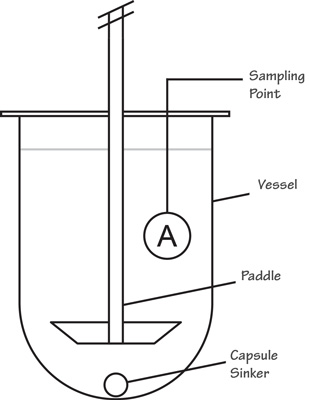
Dissolution Procedure :
The general procedure for a dissolution involves a liquid known as Dissolution Medium which is placed in the vessels of a dissolution unit. The medium can range from degassed or sonicated deionized water to pH adjusted chemically-prepared solutions and mediums that are prepared with surfactants. The dissolution medium should be water or water-based having a pH in the range of 5-7 at 37 ºC and medium to be used as per the product which is written in the standard test procedure of the respective product.
Degassing the dissolution medium through sonication or other means is important since the presence of dissolved gases may affect results so the drug is placed within the medium in the vessels after it has reached sufficient temperature and then the dissolution apparatus is operated.
Sample solutions collected from dissolution testing are commonly analyzed by HPLC and Ultra violet visible spectroscopy.
There are criteria known as release specifications that samples tested must meet statistically, both as individual values and as average of the whole and one such criteria is the parameter “Q”, which is a percentage value denoting the quantity of dissolved active ingredient within the monograph of a sample solution.
If the initial sample analysis, known as S1 or stage 1 testing fails to meet the acceptable value for Q, then additional testing known as stage 2 and 3 testing is required. S3 testing is performed only if S2 testing still fails the Q parameter. If there is a deviation from the acceptable Q values at S3, then an OOS (Out of Specification) investigation is generally initiated.
Nice post. I learn something new and challenging on blogs I stumbleupon everyday. It’s always useful to read through content from other writers and use something from their websites.
LikeLike
Thanks dear
LikeLike